The Approach
Manufacturing is much more than having a suite available. We believe that manufacturing success is driven first and foremost by proper preparation. We use a systematic internal process to ensure that protocols, batch records, raw materials, logistics, and personnel are in place well in advance of healthy donor or patient cGMP batch execution.
Prepare
- Tech transfer plan
- Electronic Batch Record (EBR)
- Project timelines and milestones
Kit
- Bill of Materials/Raw material procurement
- Bill of Equipment/Equipment install and qualification
- Disinfect and kit materials by unit operation
Qualify
- Qualify suite (EMPQ)
- Qualify analytical methods
- Train and certify staff
- Aseptic process simulation
Produce
- Engineering batches
- Clinical readiness runs
- Parallel processing
cGMP Production
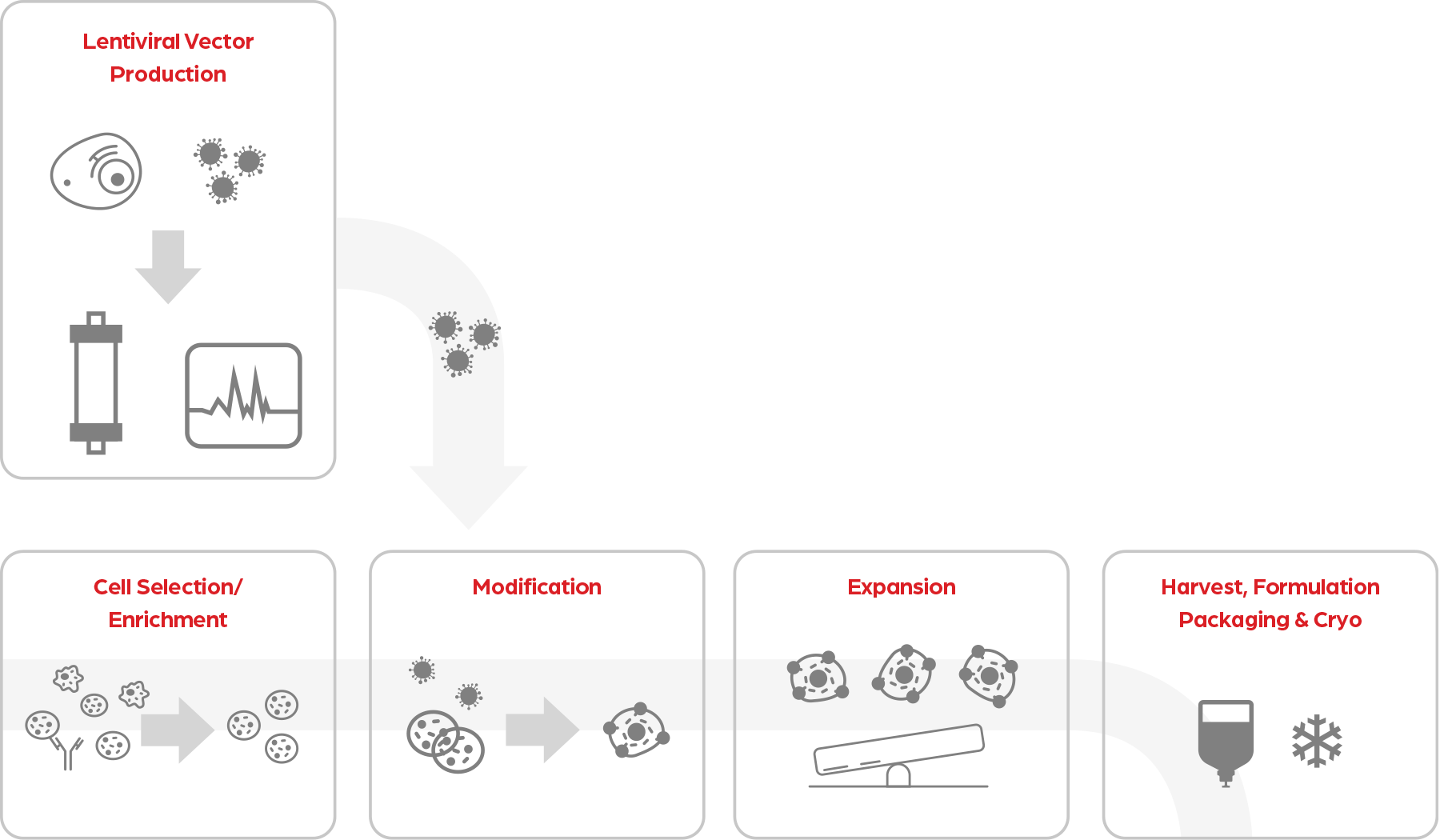
Our state-of-the-art 26,000 ft2 Arcadia manufacturing facility includes 1000 ft2 ISO 7 validated manufacturing suites that are outfitted with biosafety cabinets, incubators and all the equipment required for your process. Our production equipment is matched to our process development lab to ensure seamless transfer from development to the GMP. From pre-clinical to commercial scale, we have experience producing cells from a variety of starting materials. Our deep understanding across a range of modalities and clinical applications is what truly sets us apart, whether your working volume is 0.5 L or 100 L. Drawing upon our collective past of producing T-cells, NK cells, iPSC’s, and more, we can rapidly scale-up and release your clinical material so you can focus on your priority – providing life-changing product to the patient.
In-House Analytics
Time is of the essence with cGMP batches. Waiting for analytical test results from external laboratories leads to longer delivery times for clinical material and program delays. This is why we have heavily invested in our own in-house microbiological and analytical testing capabilities. We offer raw material, in-process, and batch release testing in-house, providing a faster batch turnaround time that can save multiple days or even weeks. Additionally, both standard assays and custom assay development are available.